Last week, we looked at how engineers and inventors took the first steps towards internal combustion, moving on from the age of steam. Several concepts led to where we are now, however, and none played a more important part in the creation of the internal combustion engine than the discovery and understanding of thermodynamics.
Remember we discussed Reflections on the Motive Power of Fire by Sadi Carnot, a breakthrough in 1824 that was often seen as one of the starting points of the second industrial revolution, through internal combustion engines?
Let’s delve a little deeper into this transition. In the 1820s, only a few inventors started thinking about the idea of directly converting combustion gas heat into mechanical energy inside a cylinder or volume.
These first steps were mainly based on empirical and experimental arguments, mainly to counter the complexity, cost and the risks around boiler explosions related to steam. As already mentioned, these genius pre-inventors – as we might call them – already identified and understood some fundamentals of the coming theory.
During the same period, the idea of replacing water steam with air as a working ‘fluid’ based on its own thermodynamic properties – independently from its ability to chemically participate in the combustion – was also driven through air-powered external combustion engines (hot air engines or ‘caloric engines’).
For a given volume, air has a lower heat capacity than steam. This means that for a given volumetric expansion, and thanks to an important drop in temperature, air is able to provide more relative work/mechanical energy than steam.
The most famous concepts were developed by Robert Stirling and John Ericsson, who already introduced air pre-compression before being heated in his first engine in 1833.
This Ericsson cycle of 1833 was the direct precursor to the Brayton-Joule cycle, which applies to gas turbines (power turbines, turboshafts and turbojets), which are part of internal combustion engines. To be perfectly fair, this cycle had already been invented in 1791 by a certain John Barber.
Bringing together the elements for the birth of the internal combustion engine
So, what do we know about these elements which, after 1850, saw the real and definitive birth of internal combustion engines?
- Different physicists from the early 19th century started studying the properties of gas, which will later become the first milestones of a new branch of physical science: Thermodynamics, or the relation between heat and other forms of energy.
- Steam engines required more and more coal, and emitted more and more smoke, but gave more and more power to their owners.
- Steam engines were seen as large, complex, expensive and dangerous (boiler explosions).
- Physicist Sadi Carnot explained and rationally synthesized the fundamentals of this future transition in Reflections on the Motive Power of Fire.
- Carnot identified and recognized the technical advances that the English had over the French at the time (remember, this was just after the Napoleon period).
- He was especially influenced by the work of James Watt.
- He was well aware of the Niépce’s Pyréolophore internal combustion engine.
- Carnot also received a high-level theoretical training from Ecole Polytechnique in Paris, being in contact with many of these precursors of thermodynamics.
- He correctly analyzed and understood the limits of the steam machines.
During the first period after its publication, sales of Carnot’s Reflections on the Motive Power of Firewere not particularly widespread. However, it was noticed by one of the first thermodynamicists, Emile Clapeyron.
In his memoir, Clapeyron presented Carnot’s work in a more accessible and analytical graphical form, showing the Carnot cycle as a closed curve on an indicator diagram, a chart of pressure against volume, with a chart eventually named Clapeyron’s graph in his honor. Clapeyron’s analysis of Carnot was further and more broadly disseminated in 1843, when Johann Poggendorff translated it into German.
In 1842, Clapeyron published his findings on the ‘optimal position for the piston at which the various valves should be opened or closed’. In 1843, Clapeyron further developed the idea of a reversible process, already suggested by Carnot, and made a definitive statement of Carnot’s principle, which is now known as the second law of thermodynamics.
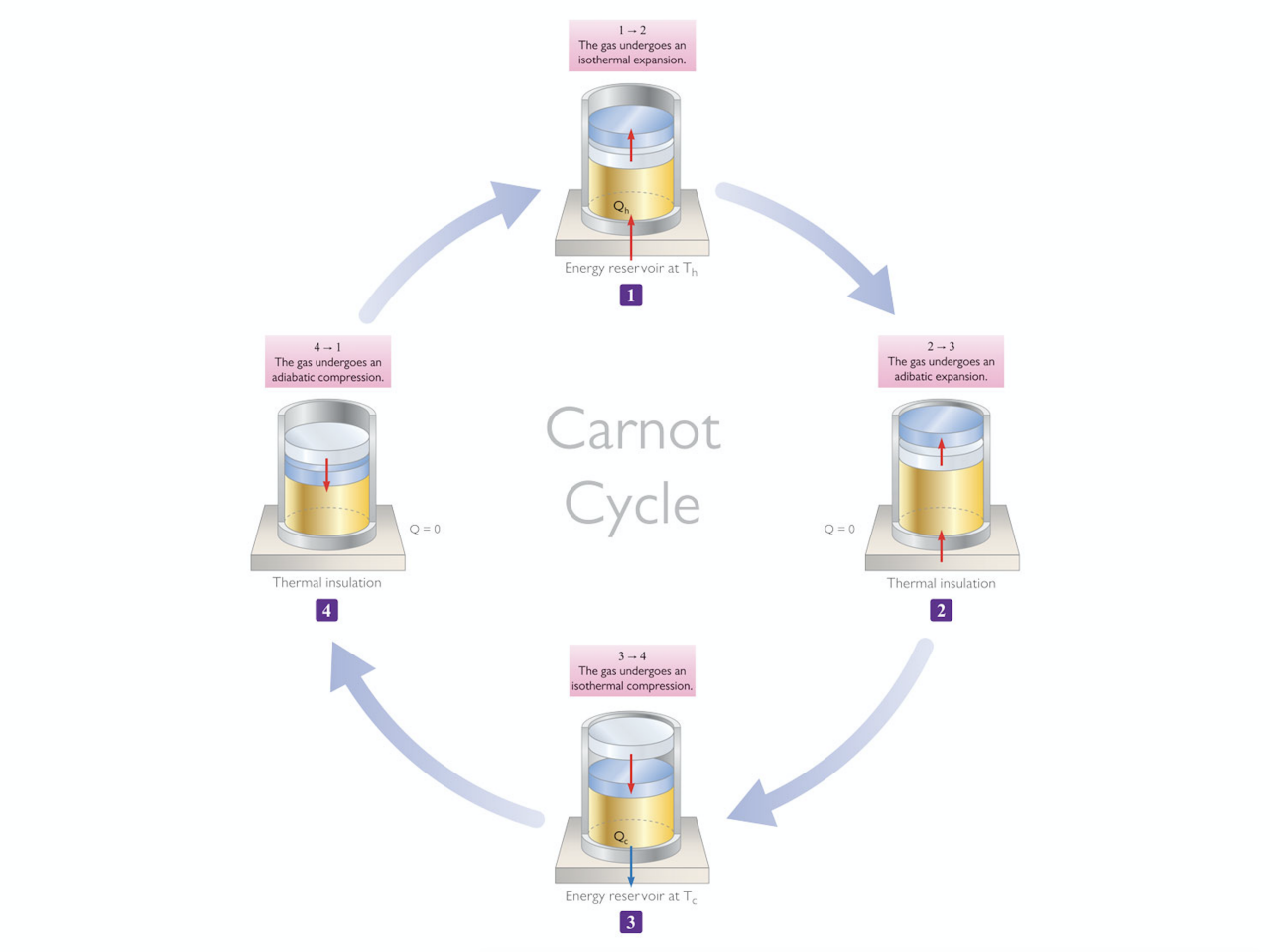 The Carnot Cycle
The Carnot Cycle
Becoming a staple ingredient in engineering in the mid-19th century
The work was finally embedded into engineers’ knowledge mainly when the extremely important and international work of Rudolf Clausius and Lord Kelvin made its true significance generally known.
Here’s what Carnot wrote, that would change engineering forever, in simple words:
- The ratio between maximal working fluid temperature and cold source temperature (often ambient temperature) may be the highest as possible.
- For a given set of maximal and cold temperatures – respectively Th and Tc – the best efficiency is obtained with following ideal cycle:
1-2: Isothermal Expansion at Th
2-3: Adiabatic Expansion
3-4: Isothermal Compression at Tc
4-1: Adiabatic Compression
(Adiabatic = without any heat exchange with external environment)
And that’s the point we reached during the mid-19th century, around 10-15 years before Lenoir’s main invention. We’ll be taking a closer look at that soon in the next installment of the history of the internal combustion engine…